Cure SMA Releases Update on Spinal Muscular Atrophy Drug Pipeline
Written by |

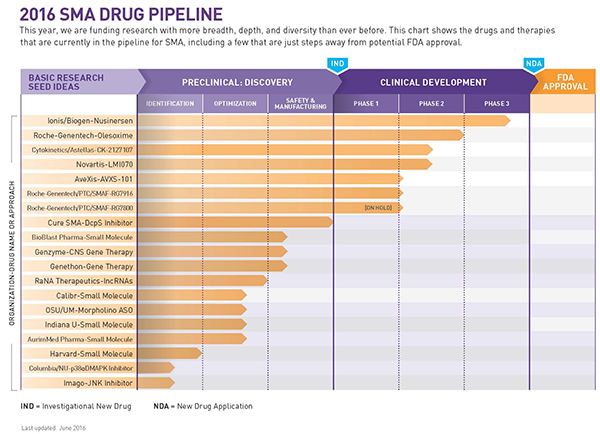
Cure SMA has just released an update on its spinal muscular atrophy (SMA) drug pipeline and, according to a press release, the latest version now includes increased coverage of potential treatment approaches to SMA: 18 active programs, 14 pharmaceutical partners, six programs in clinical trials, and 28 programs in the cumulative pipeline total, including 10 failures to date.
Recent research has made critical progress, such as finding a series of systems, pathways, and processes that have been shown to be affected by SMA. These findings might point toward new ways to treat this devastating disease and, more importantly, possibly reveal new approaches that could work on changing SMN levels, allowing the medical community to attack SMA from different fronts and opening more doors for potentially comprehensive, effective treatments.
Since individuals with SMA don’t produce enough survival motor neuron (SMN) proteins due to an SMN1 gene mutation, many of the programs available today rely precisely on these SMN-based approaches like gene therapy or antisense oligonucleotides. Previous research has mainly focused on increasing SMN production – either by replacing SMN1 or by modulating SMN2, the low-functioning SMN “back-up gene.”
The Cure SMA pipeline shows that a few of these approaches are already being evaluated in clinical trials. In the future, these approaches could potentially be used alone or in combination with other therapies. In the next several weeks the organization will also announce funding for ongoing programs in the SMA pipeline focused on improving the SMN2 gene functioning.
As the SMA drug pipeline expands and grows in diversity and complexity, the need for multiple therapeutic approaches and combination therapies has become increasingly apparent. In the last six months, since its last pipeline update in December 2015, Cure SMA has added two new SMA programs, funded by grants from the organization itself, both researching and developing combination therapies.
The organization recommends that despite these improvements, more work needs to be done, noting that only 10 percent of the drugs that reach clinical trials are ultimately granted U.S. Food and Drug Administration (FDA) approval.
Beyond funding the pipeline, Cure SMA has made a commitment to advocate for patients in the development of drugs to ensure regulators understand the needs of the SMA community.