FAQs about SMA causes
The most common types of spinal muscular atrophy (SMA) are caused by mutations in the SMN1 gene. Specifically, a deletion of exon 7, in which a specific section of the SMN1 gene is missing from the genetic code, is the most common SMA-causing mutation, accounting for about 94% of cases.
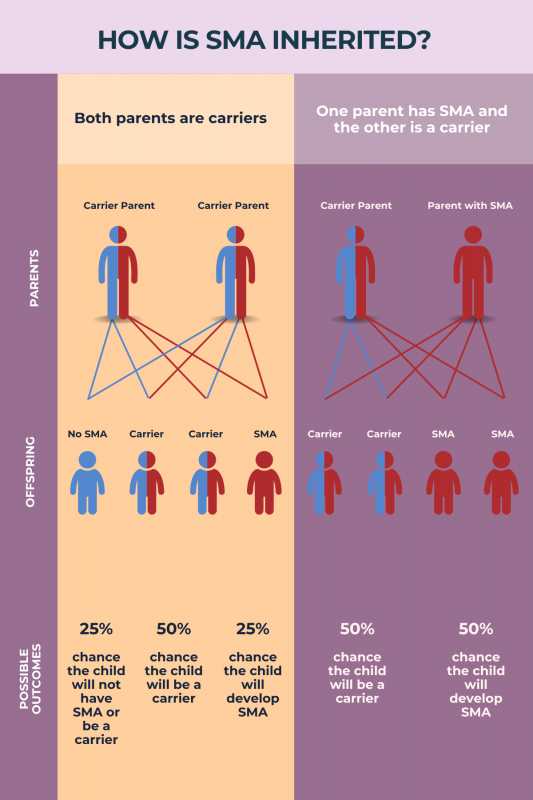
Spinal muscular atrophy (SMA) is a genetic disorder caused by mutations that are present from the moment of conception. Inherited SMN1 gene mutations are the main cause of SMA, but rarer forms of the disease are caused by mutations in other genes. In the majority of cases, offspring will develop the disease if they inherit one mutated gene copy from each of their biological parents.
The main forms of spinal muscular atrophy (SMA), caused by mutations in the SMN1 gene, only develop if a child inherits two mutated copies of the gene (one from each biological parent). This means that a child will only develop SMA if they are born to either two people with SMA, or a patient and a carrier. However, some rarer forms of SMA have distinct inheritance patterns and may develop if the child inherits only one mutated copy from one of their parents.
The five main types of spinal muscular atrophy (SMA) only develop if a person inherits two mutated copies of the SMN1 gene. If only one parent is a carrier, the child can only inherit one mutated copy and it is very unlikely he or she will develop SMA. However, in extremely rare cases, a mutation can develop spontaneously in a sperm or egg cell, so the disease can develop in a child evenif they only inherited one mutated copy from the carrier parent.
The mutations that cause spinal muscular atrophy (SMA) are present from the moment that a sperm cell fertilizes an egg cell. Lifestyle choices cannot cause these genetic mutations, but some unhealthy habits, such as having a sedentary lifestyle, can increase the risk of complications in people with the disease.
Related Articles

 Medically reviewed by
Medically reviewed by