FAQs about SMA
There is no spinal muscular atrophy (SMA) cure. However, there are several approved therapies that can slow the progression of SMA and offer a better quality of life for people with the disease.
Spinal muscular atrophy (SMA) is a progressive disease, meaning symptoms typically get worse as time goes on. There are multiple medications that have been proven to slow or even stop the progression of the main types of SMA. Apart from such medicines, other medical care strategies, including specialized equipment or physical therapy, can be of benefit to people with SMA and help make day-to-day life easier.
Life expectancy for spinal muscular atrophy (SMA) varies by disease type — babies with the most severe forms of SMA usually don’t survive past infancy without treatment, while milder forms of the disorder do not usually affect life expectancy. Modern treatments have been shown to radically improve SMA life expectancy outcomes in severe disease types. However, because these therapies were only developed in the past decade, the long-term life expectancy for someone given modern treatment is still unknown.
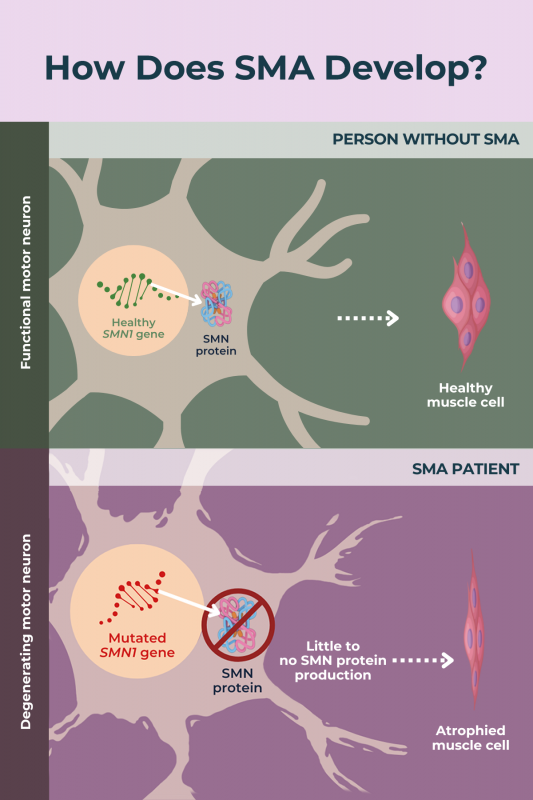
The gold standard for diagnosing spinal muscular atrophy (SMA) is genetic testing to identify disease-causing mutations. This testing can be done at any point in life, and it is even possible during pregnancy. It is recommended that babies be tested for SMA if there is a known family history of the disease, or if they show symptoms indicative of SMA, including muscle weakness, floppy limbs, difficulty feeding, trouble breathing, and failure to hit motor milestones like rolling over, sitting up, or crawling.
According to the National Organization for Rare Disorders, spinal muscular atrophy (SMA) affects approximately 1 of every 10,000 live births, and the SMA Foundation estimates that about 10,000-25,000 children and adults in the U.S. have the disease. Because it affects fewer than 200,000 people in the country, SMA is considered a rare disease.
Related Articles

 Medically reviewed by
Medically reviewed by