SMA Patient Muscle is Deficient in Mitochondrial Biogenesis
Written by |
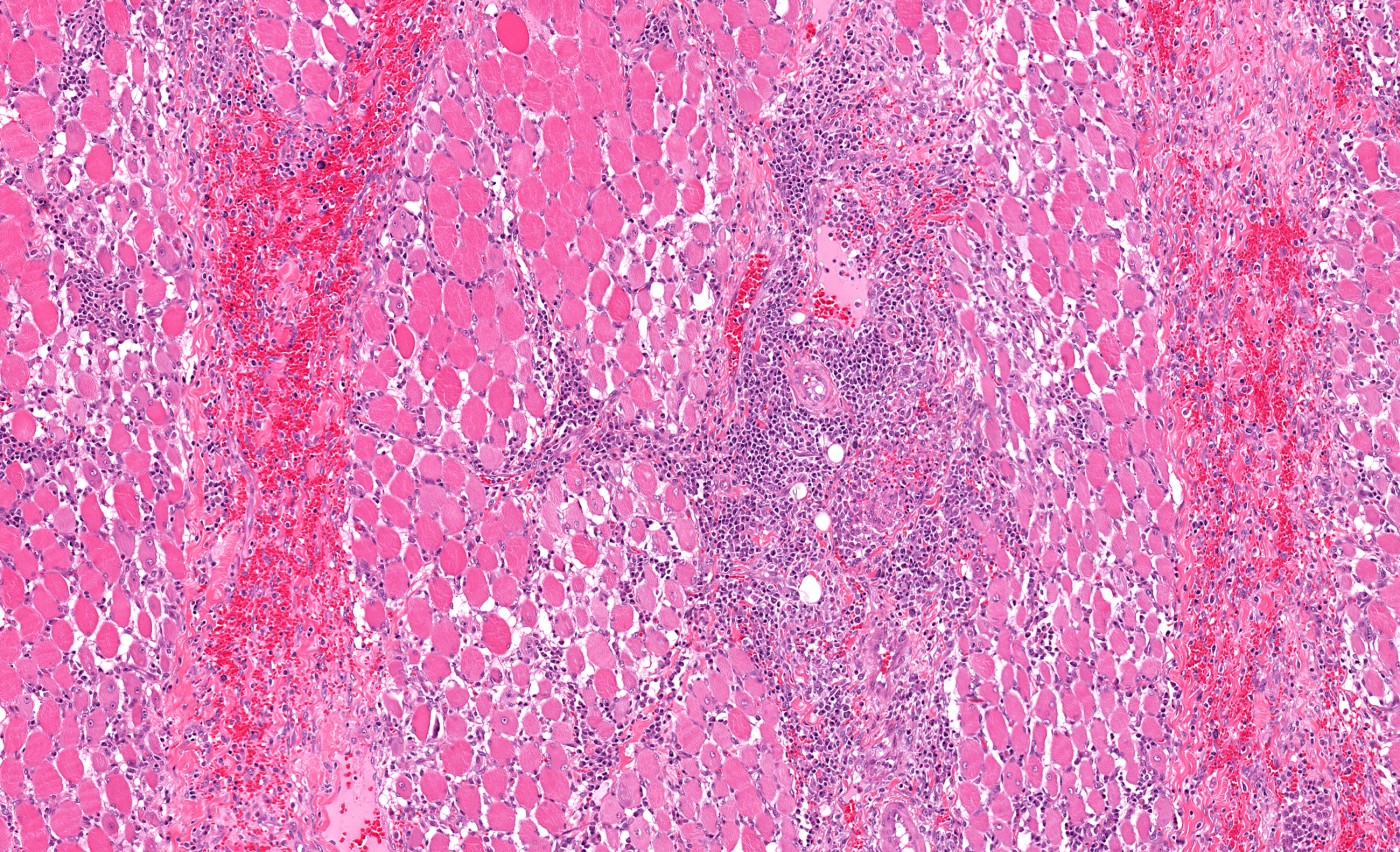
 Patients with spinal muscular atrophy (SMA) exhibit many signs of mitochondrial paucity. Weakened muscles and other pathologic muscle changes are accompanied by decreased levels of mitochondrial DNA (mtDNA) and decreased levels of mitochondrial respiratory chain complex proteins, such as complex II. Although all signs lead to decreased levels of mitochondria, it was not confirmed until a research group in Italy conducted a study entitled “Impaired Muscle Mitochondrial Biogenesis and Myogenesis in Spinal Muscular Atrophy,” which was published in JAMA Neurology.
Patients with spinal muscular atrophy (SMA) exhibit many signs of mitochondrial paucity. Weakened muscles and other pathologic muscle changes are accompanied by decreased levels of mitochondrial DNA (mtDNA) and decreased levels of mitochondrial respiratory chain complex proteins, such as complex II. Although all signs lead to decreased levels of mitochondria, it was not confirmed until a research group in Italy conducted a study entitled “Impaired Muscle Mitochondrial Biogenesis and Myogenesis in Spinal Muscular Atrophy,” which was published in JAMA Neurology.
“Our results strongly support the conclusion that an altered regulation of myogenesis and a downregulated mitochondrial biogenesis contribute to pathologic change in the muscle of patients with SMA,” wrote lead author Michela Ripolone, PhD.
The team of researchers attained their results using muscle samples obtained from 28 patients with confirmed genetic SMA. Three were paraspinal muscle samples, 24 were quadriceps muscle samples, and one was a postmortem sample. To compare, the researchers also obtained muscle biopsies from patients between one and three years of age who had suspected myopathy but were healthy.
Muscles were analyzed using histochemical staining for cytochrome-c oxidase (complex IV) and tested for residual activity of complexes I, II, and IV. “Cytochrome-c oxidase (COX) deficiency was more evident in muscle samples from patients with SMA-I and SMA-II,” described Dr. Ripolone. “Residual activities for complexes I, II, and IV in muscles from patients with SMA-I were 41%, 27%, and 30%, respectively, compared with control samples.”
[adrotate group=”3″]
In accordance with previous studies, the researchers also detected lower levels of mtDNA in muscle and reduced cytrate synthase enzymatic activity in SMA patients, likely due to a downregulation of peroxisome proliferator-activated receptor coactivator 1α, the transcriptional activators nuclear respiratory factor 1 and nuclear respiratory factor 2, and mitochondrial transcription factor A — all of which are regulators of mitochondrial biogenesis.
Contrasting these deficiencies, muscle from SMA patients had an increased level of expression of muscle-specific myogenic factor 5, myoblast determination 1, and myogenin. These are three myogenic regulatory factors involved in muscle development and repair. It is not surprising to see an increase in markers for repair, as SMA patients generally are affected by muscle atrophy and degeneration.
Dr. Ripolone notes that the aim of the study was to help other researchers find targets for new SMA treatments. “Therapeutic strategies should aim at counteracting these changes,” she wrote.



