Stella’s ‘Very Comfortable’ Hour with Zolgensma and Pleas for Newborn Screening: The Lackeys on Their SMA Journey
Written by |

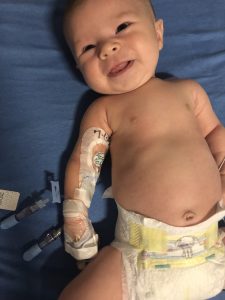
Stella resting after her hourlong infusion with Zolgensma on July 3. (Photo courtesy of Lackey family)
[Editor’s Note: In the conclusion of this two-part interview with the Lackey family of Arizona about their daughter’s treatment with Zolgensma at 6 weeks old, we look at treatment day, advice they have to make it easier, and the family’s decision to advocate for screening newborns for SMA.]
Samantha and Jeremy Lackey heard the worst news that young parents could possibly hear — their daughter had spinal muscular atrophy type 1, fatal if untreated — on June 18, the day Stella turned 1 month old.
Within a week, on June 23, they were informed their girl had been accepted — insurance coverage and all — for treatment with Zolgensma, a newly approved gene therapy that held the promise of being a one-time and life-saving treatment.
“We can’t even speak to how grateful we are for how quickly things went and how everyone was so supportive and responsive, giving us the answers we needed and helping us to make the decisions we needed to make,” Jeremy Lackey told SMA News Today in a recent telephone interview from his Arizona office.
“I know a lot of that has to do with the fact that it’s new and it’s different and cutting-edge, for lack of a better term,” he added. “And it goes to the fact that every day matters; having the opportunity to treat it as quickly as we did is going to bode well for us in the long run.”
Stella’s big day, July 3, was as easy as it was memorable.
Receiving Zolgensma
Zolgensma, developed and marketed by AveXis and its parent company Novartis, retails for $2.125 million. Not surprisingly, Samantha’s first reaction to learning the treatment request had been approved by her insurance carrier, Cigna, was to confirm that with the company.
“Through the whole process, Cigna has been fantastic,” Samantha said, detailing not only the approval, but an array of help offered by her insurer during and after treatment. “Another thing we want to stress: We are incredibly lucky to have the insurance we have.” Good coverage “also helps this process go so much faster,” she said.
Time is crucial in SMA, because the motor nerve cells damaged due to the disease-causing mutations in the SMN1 gene die quickly. And motor neurons do not regenerate; what’s lost is gone forever.
July 3 started early with Stella brought to the Center for Cancer and Blood Disorders at Phoenix Children’s Hospital for her hour-long intravenous (IV) infusion.
A patient resource manager and others with AveXis’ OneGene Program, created to guide SMA families through to Zolgensma treatment, met the Lackeys there. They waited outside the “very small” treatment room, while Stella went through a basic checkup followed by the infusion.
“She slept the whole time … was very comfortable,” Samantha said of the Zolgensma process. A “fantastic” nurse stayed with the family throughout, checking the baby’s vital signs every 15 minutes.
“We were able to hold her; she was not bothered by any of it,” her mother added.
A possibly key decision was having a PICC line inserted two days earlier. Stella’s neurologist, Saunders Bernes, at Phoenix Children’s, recommended a PICC because of the difficulties in putting an IV into an infant; it’s a “painful and annoying” process given a baby’s small veins, Jeremy said. Doing it in advance made PICC day a horror — Stella “went under anesthesia, which was stressful for us” — but treatment day “much easier,” he added.
Instead of searching for a vein to place the IV, the infusion was done with a quick screw-in to the PICC’s port that let the infusion “drip off. … The PICC line has made Stella’s life easier, it’s made our life easier,” Jeremy said.
Stella still has a PICC in her right arm, which the Lackeys don’t plan to remove for a month until blood tests — of liver enzymes, platelet counts and other post-treatment safety checks — are done and she’s cleared.
“It’s an easy flush the line and get the blood out of it” for Stella’s weekly blood checks, done each Tuesday through early August. “It’s not having to put a needle into her arm and draw blood out,” Jeremy said. “Just having the PICC line is going to make everything more comfortable.”
To date, the only complication since Stella’s return home was a fever that developed two days’ post-treatment. The baby, born May 18, was hospitalized and the fever, which peaked at 102°F (38.8°C), dropped within 24 hours with fluids and Tylenol as treatment.
In the less than two weeks since getting Zolgensma, Stella’s cries have become louder, “like someone turned up the volume,” her mother said. “We’re hesitant” to say more because it’s all so new, “but she definitely seems to be breathing a lot easier, and yes, we have seen more movement. … It’s pretty incredible, actually.” A video on the baby’s Instagram page shows her grasping a ball on her chest, accompanied by the words: “Stella isn’t able to bring the ball to her mouth just yet. … We’ll get there.”
Final costs of treatment aren’t known because many bills have yet to arrive. “But we do know that we have met our deductible, rather quickly,” Samantha said. “Stella and I both met our deductible.
“I almost really haven’t had time to fully wrap my head around it all,” she added.
Looking ahead, with determination
For the Lackeys, using a gene therapy did not merit a second’s hesitation.
“We can’t use the word cure,” Samantha said, “but at least having the opportunity to try and attack it [SMA], to fight it, we felt was absolutely in our best interest.” Unlike Spinraza (nusinersen, by Biogen), which works to treat SMA by manipulating a secondary gene, SMN2, so it produces a full-length and working SMN protein, Zolgensma works by delivering a functional copy of the disease-causing SMN1 gene most responsible for producing that protein. Repeat clinical trials have shown it highly effective in very young patients.
Given that Stella was about 6 weeks old at treatment, it made the most sense, both parents agreed.
Stella was diagnosed partly because her pediatrician was observant and careful, testing the baby’s reflexes at a visit prompted by a blocked tear duct. And that doctor was willing to reconsider what treatment Stella most needed when he didn’t like what he saw in her reflexes.
It also was due to Samantha going through genetic screening in the first trimester of her pregnancy, because her mother had been adopted and her family’s medical history not fully known. Results showed she was an SMA carrier (and a carrier for cystic fibrosis and cholestasis), and Samantha faithfully followed her obstetrician’s advice, alerting each of Stella’s pediatricians that she could pass along the disease.
SMA is autosomal recessive, meaning that both parents need to be carriers for a child to inherit it. Jeremy was not screened; neither parent knew of a family history of SMA. But Samantha’s notice was enough to catch a pediatrician’s attention.
“We know that we got very, very lucky,” Jeremy said repeatedly in the SMA News Today interview. “Everything worked out in our favor. We had so many people in our corner who were making things happen for us.”
The Lackeys well know things could have been so much different, and often are for SMA children.
Stella’s case, and the speed at which her SMA was detected, confirmed, and the treatment process started and completed — 16 days in total from diagnosis to Zolgensma infusion — is not yet, in any sense, a norm for patients or their families.
That’s why the Lackeys want to be advocates. They want to help other families learn from their experiences and repeat the best of them — reaching out for information, connecting with other families through social media and groups like Cure SMA, choosing a PICC line, and trusting their instincts.
And they want SMA families, and all others touched by or concerned about this disease, to pester lawmakers to ensure that SMA is added to newborn screening programs nationwide.
Only seven states currently test babies at birth for SMA, despite both Spinraza (approved in December 2016) and Zolgensma (approved May 24, 2019) being available as disease-modifying treatments. Another 16 have either passed laws requiring such screening, or are in the process of doing so.
Federal efforts to push for broader screening lack financial support, the Muscular Dystrophy Association told SMA News Today in a May interview, and individual states can be torn between conflicting priorities, especially for unfunded or underfunded demands.
That’s why advocating is so important, Jeremy and Samantha said. Little is likely unless everyone concerned writes or calls their lawmakers — senators and representatives at state and federal levels — to demand that SMA be added to the dozens of diseases already screened for in blood samples from newborns.
“It’s a loaded statement in that it’s so easy — just get your senator or local representative on the phone and voice your concern,” Jeremy said. “But you’re one, and Samantha and I are two, out of millions.
“The more people who are writing for and speaking of newborn screening, the more likely it is to move forward. And specifically with SMA, the earlier you catch it and the earlier you treat it, the better. The more hope for the future there is.”
[Editor’s note: Those wishing to follow Stella’s journey with SMA and Zolgensma may do so here in the coming months, and via Stella’s page on Instagram, stronglikestella, where her story is being documented in words, photos, and videos.]