Discussion
Discussion
FAQs about Evrysdi
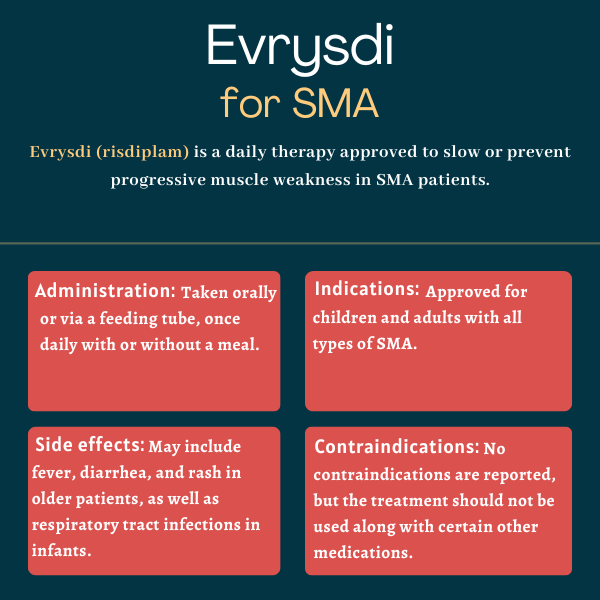
The U.S. Food and Drug Administration first approved Evrysdi in 2020 for the treatment of adults and children, ages 2 months and older, with any type of spinal muscular atrophy (SMA). This marked the third approval of a disease-modifying drug for the disease in fewer than four years. That indication was later expanded in 2022 to make Evrysdi available in the U.S. to patients of all ages.
Evrysdi can help maintain or improve motor function in people with spinal muscular atrophy, aiding them in attaining milestones that normally would not be reached in untreated patients. The exact timing for observing a benefit from this treatment will vary considerably depending on a person’s disease type, age at treatment initiation, and other individual characteristics, but most trials of Evrysdi have reported significant benefits from the therapy after one year of treatment.
In patients with later-onset spinal muscular atrophy (SMA), including children and adults ages 2 and older, the most common side effects from Evrysdi in clinical trials were fever, diarrhea, and rash. In infantile-onset SMA, the side effects were similar to those reported in older patients but also included respiratory tract infections, constipation, vomiting, and cough. The safety profile was similar in patients treated with Evrysdi before their symptoms were evident.
No contraindications or long-term complications are listed in Evrysdi prescribing information in the U.S. Yet, patients with spinal muscular atrophy (SMA) taking the medication can still experience complications associated with the progression of the disease, including respiratory problems and trouble swallowing.
Yes, Evrysdi is now approved in more than 100 countries for both children and adults with all forms of spinal muscular atrophy. While younger patients, who have less extensive neuronal damage and muscle weakness, tend to draw more significant benefits from Evrysdi, the therapy also can help adults by maintaining motor function or slowing its decline.
 Fact-checked by
Fact-checked by