Novel SMA Research Seeks to Correct SMN Protein Production
Written by |
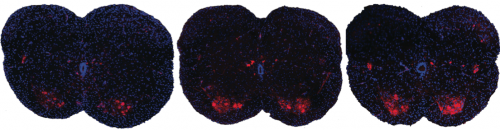
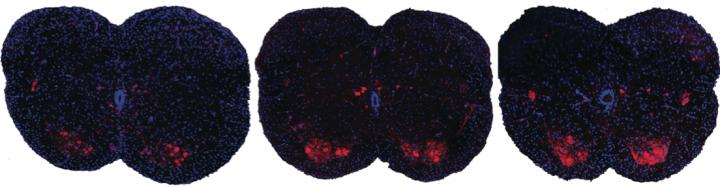
Shown here are spinal sections from three different mice with spinal muscular atrophy. Systemic drug treatment (middle panel) increases the presence of motor neurons (red spots) over the untreated mice (left panel). Surprisingly, the results are very similar when treatment is excluded from the central nervous system (right panel), suggesting a possible new path for spinal muscular atrophy drug treatment.
CREDIT
A. Krainer/ Cold Spring Harbor Laboratory
Cold Spring Harbor Laboratory (CSHL) researchers announced an advance regarding the possibility of a drug treatment for spinal muscular atrophy (SMA) in the recent study “Motor neuron cell-nonautonomous rescue of spinal muscular atrophy phenotypes in mild and severe transgenic mouse models.” The findings, published in the journal Genes & Development, may help establish better strategies for the treatment of SMA patients.
SMA is the leading genetic cause of death in infants worldwide. It is a rare, devastating motor neuron disease, characterized by the degeneration of nerves controlling muscles and voluntary movement, resulting in paralysis and eventually death. SMA has no approved treatment.
Neurons need a protein named Survival of Motor Neuron (SMN), which has two variants – SMN1 and SMN2. SMN1 is abundant in healthy individuals, while SMN2 is produced mainly as an unstable and shortened form. In contrast, in SMA patients, cells have a defect in the SMN1 gene and do not produce SMN1 protein, instead, they depend on the minor amount of full-length SMN2 protein that is produced together with the shorter, non-functional SMN2 form.
In a collaboration with Professor Adrian Krainer, senior lead author of the study, and Isis Pharmaceuticals, a drug was developed that allows correct editing of SMN to significantly increase the expression of functional SMN2 protein. This drug, named antisense oligonucleotide (ASO), exhibited promising results in preclinical tests using SMA mouse models and it is currently in Phase 3 clinical trials in SMA patients.
The ASO drug is directly injected into the cerebrospinal fluid of SMA children. “It is largely believed in our field that SMN is essential in the central nervous system (CNS) – not in peripheral tissues like the limbs or liver – so most efforts have been focused on increasing full-length SMN levels in the CNS,” explained Krainer. “But over the last few years, evidence has been building that challenges our assumptions about the pathology of the disease. The question is: do we need to increase SMN levels in the CNS, in peripheral tissues, or both?”
[adrotate group=”3″]
To answer this question, the team developed a technique to increase SMN levels only in the peripheral tissues. SMA mouse models were injected subcutaneously with the ASO drug and the results showed that the drug improved SMN protein levels in both peripheral tissues and CNS, especially in newborn mice. The treatment was effective and the animals were cured.
The team used a “decoy” oligonucleotide (to block the action of ASO) that they have injected directly in the CNS, together with a subcutaneous injection of SMA ASO drug as before. Krainer explained the results “Our decoy was thus able to inactivate the drug only in the CNS”. “Full-length SMN2 was still produced in peripheral tissues, but there was no increase in SMN2 made in the CNS, where we expected it to be important.”. “We were amazed to find that our decoy had absolutely no effect on motor neurons or recovery. Contrary to our initial assumptions, increasing SMN levels in the CNS is not essential to rescue the SMA phenotypes in these mouse models of the disease.” Such unexpected results lead the researchers to postulate that at least to be effective in mice, the drug does not have to be delivered directly into the CNS, increasing the SMN levels exclusively in the peripheral tissues rescued the phenotype, improved motor function and extended survival in severe SMA mice. This observation open ups a new path for SMA drug treatment.
“Already, our ASO is showing great potential in the clinic,” said Krainer. “There is a possibility that modifying the way the drug is administered may produce even better outcomes; alternatively, our observations may reflect unique idiosyncrasies of the mouse models, but even then it will be important to understand the underlying mechanisms.”



