Biogen acquires Alcyone, device aimed at improving drug delivery
ThecaFlex DRx System being evaluated with Spinraza in SMA patients
Biogen has acquired Alcyone Therapeutics and will continue to develop ThecaFlex DRx, a device intended to ease patient experience and accessibility when delivering certain medicines into the spinal canal.
The ThecaFlex DRx System is initially being evaluated with Spinraza (nusinersen) in people with spinal muscular atrophy (SMA), which Biogen says will inform strategies for the company’s investigational therapies.
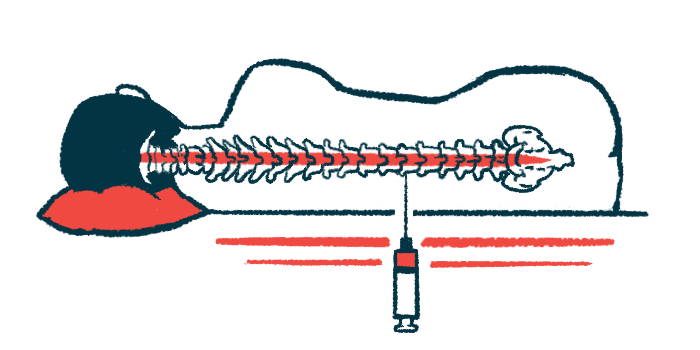
The implantable, subcutaneous (under-the-skin) port and catheter device is specifically being investigated for the intrathecal (spinal canal) delivery of antisense oligonucleotides (ASO). ASO therapies such as Spinraza bind to RNA.
The device is aimed at providing a convenient alternative to repeated lumbar punctures, or spinal taps. In a one-time surgery, clinicians implant a catheter into the spinal canal that connects to a port under the skin for injection.
In 2023, Biogen partnered with Alcyone to develop the ThecaFlex DRx System as a delivery option for ASO therapies in certain neurological disorders.
“For nearly three decades, Biogen has pioneered ASO development, and we are committed to continuing to improve patient experience and ease of administration,” Nicole Murphy, head of pharmaceutical operations and technology at Biogen, said in a company press release. “We believe the acquisition of Alcyone Therapeutics offers a strategic opportunity to both expand the company’s capabilities and enhance the value proposition of our medicines by offering a meaningful, patient-centered solution.”
Biogen will oversee development of drug delivery device
Under the agreement, Biogen will buy Alcyone for $85 million upfront, with potential further payments upon reaching development and regulatory milestones.
“With ThecaFlex DRx, following our productive partnership with Biogen, we now have the opportunity to further deliver what could be the first truly patient-centered, chronic intrathecal delivery option for these important therapies,” said PJ Anand, founder, president, and CEO of Alcyone.
Following the acquisition, Biogen will oversee the device’s development, manufacturing, and commercialization.
“We believe Biogen’s deep expertise in ASOs and its proven track record in advancing drug delivery innovations make it the ideal partner to bring this technology forward,” Anand said.
2 trials to test device in SMA patients
Two clinical trials — the PIERRE study (NCT05866419) and the Phase 1 PIERRE-PK study (NCT06555419) — are testing the device in people with SMA. Both trials are currently enrolling participants with SMA ages 3 and older at sites in the U.S. and Europe. The PIERRE study is recruiting participants who are resistant to lumbar puncture and are either new to Spinraza therapy or will continue it via the ThecaFlex DRx System.
A subset of PIERRE participants on a stable maintenance dose of Spinraza will also participate in the PIERRE-PK study. These individuals will receive Spinraza by spinal tap before the ThecaFlex DRx implant. Investigators will then compare how the body processes the medication when delivered via spinal tap versus ThecaFlex DRx.
Mutations in the SMN1 gene are the chief cause of SMA, a neuromuscular condition that leads to progressive muscle weakness and other symptoms. Typically, SMN1 instructs cells to produce the survival motor neuron (SMN) protein. SMA-causing mutations lead to a deficiency in functional SMN, a protein that plays an important role in the functioning and maintenance of specialized nerve cells called motor neurons.
Another gene, SMN2, also contains instructions for producing SMN. However, a natural event called alternative splicing reduces the amount of working and stable protein that cells can produce using SMN2. Spinraza aims to increase SMN production from SMN2 by binding to the gene’s messenger RNA molecule, an intermediary made from DNA that contains instructions to make proteins, and correcting its splicing.
The U.S. Food and Drug Administration has granted breakthrough device designation to the ThecaFlex DRx System, which confers more timely feedback with the agency and prioritized review of a regulatory approval application.