Zolgensma into spinal canal drives SMA type 2 motor gains
STEER tested formulation on 100+ children who couldn't walk on their own
Written by |
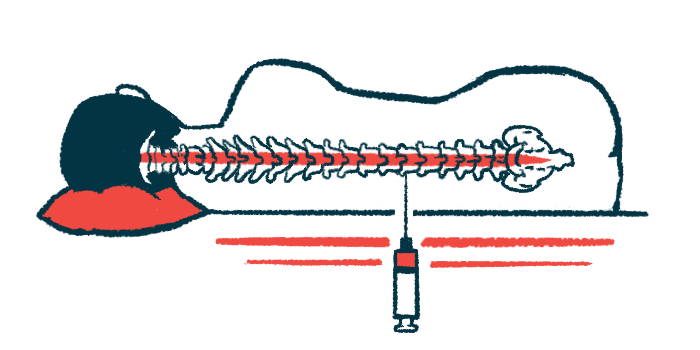
Treatment with OAV101 IT, a formulation of the gene therapy Zolgensma (onasemnogene abeparvovec-xioi) that’s delivered directly into the spinal canal, safely led to motor improvements for children with spinal muscular atrophy (SMA) type 2 who were at least 2 years old, top-line results from the Phase 3 STEER trial show.
Novartis plans to share the data with regulatory agencies, including the U.S. Food and Drug Administration, this year toward making the treatment available to SMA patients. The data will also be presented this year at a medical meeting.
“These positive top-line results from the STEER trial underscore the efficacy, safety and tolerability of OAV101 IT in patients with SMA aged 2 and above,” Shreeram Aradhye, MD, president, development and chief medical officer at Novartis, said in a company press release. “The totality of evidence clearly supports a positive risk benefit profile of OAV101, which is expected to support registration [approval] covering a broad range of SMA patients.”
Zolgensma is a gene therapy that’s designed to deliver a functional version of the SMN1 gene, mutations in which are the cause of SMA. The treatment is packaged into a viral carrier that helps it be taken up by cells when given via a one-time infusion into the bloodstream (intravenous delivery). In the U.S., Zolgensma is approved for patients with any type of SMA up to age 2.
OAV101 IT is an intrathecal formulation of Zolgensma that’s designed to be injected directly into the fluid-filled spaces in the spine. This gives the treatment more direct access to the brain and spinal cord than an via intravenous infusion, requiring a lower dose to be effective. The treatment was tested in the open-label Phase 1/2 STRONG (NCT03381729) trial, which showed the gene therapy led to clinically meaningful improvements in motor function, as assessed by the Hammersmith Functional Motor Scale-Expanded (HFMSE), for children with SMA type 2 and type 3, ages 2-5.
STEER results
The Phase 3 STEER trial (NCT05089656) was designed to build upon those results. It enrolled more than 100 children with SMA type 2, ages 2-17, who were able to sit, but had never walked independently. None had been previously treated with disease-modifying therapies.
The participants were randomly assigned to receive OAV101 IT at a dose of 1.2 x 1014 vector genomes or a sham procedure to mimic the administration but without any active treatment. A year after treatment, patients given the sham procedure could receive OAV101 IT and those who’d received the gene therapy were given the sham procedure, and monitored for three more months. The main goal was to evaluate changes in motor function, as assessed by total scores on the HFMSE, a year after treatment.
Patients treated with intrathecal Zolgensma saw improved HFMSE scores a year after receiving the gene therapy compared with before treatment, meeting the main goal. Gains seen in the treatment group were greater than in the sham group.
“Maintaining motor function is a key goal for many older patients with SMA,” said Crystal Proud, MD, a neurologist and principal investigator for the study at the Children’s Hospital of the King’s Daughters in Virginia. “This may allow them the capacity to continue to propel their electric wheelchair, feed themselves with intact hand-to-mouth function, and perform other activities of daily living as independently as possible. OAV101 IT administration has not only been demonstrated to maintain motor function, but also increased it in indicating the impact a one-time therapy could have.”
The treatment’s safety profile was also favorable, with similar rates of adverse events in the two arms, according to the company. The most common side effects were upper respiratory tract infection, fever, and vomiting.
Another open-label Phase 3b study called STRENGTH (NCT05386680) is designed to evaluate OAV101 IT in 27 SMA patients, ages 2-17, who had discontinued other SMA therapies, namely Spinraza (nusinersen) or Evrysdi (risdiplam). Its main outcome measure is safety, with other functional assessments as secondary trial goals.