How Does Nusinersen Work in Treating SMA?
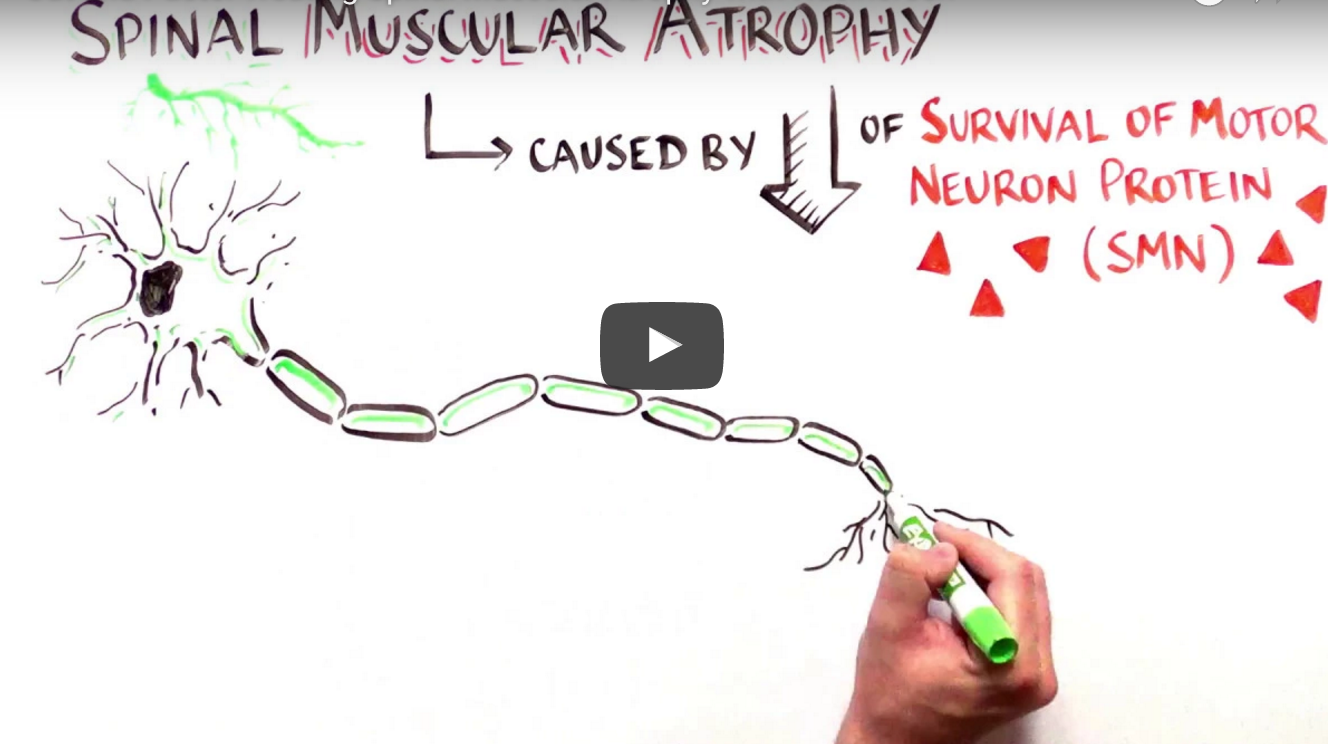
This video from Youreka Science explains exactly how the newly FDA-approved drug, nusinersen, works in helping children with spinal muscular atrophy (SMA).
Nusinersen found safe and effective in treating infants with SMA Type 1.
The film explains the genetic defects which lead to SMA, the faulty genes involved and how nusinersen enables patients to make more of the vital SMN protein.
Nusinersen was approved by the Food and Drug Administration (FDA) in December 2016 to treat babies, children, and adults with the disease. Clinical trials showed promising results with many babies with type 1 SMA reaching milestones such as rolling, crawling, head control, standing and walking–milestones usually not met by children with the worst type of the disease. Find out more about the FDA’s approval of nusinersen here.
Find out about Cameron’s journey on the clinical trial for nusinersen.
SMA News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.







