Spinal Surgery Shouldn’t Preclude Access to Spinraza, Polish SMA Expert Argues
Written by |
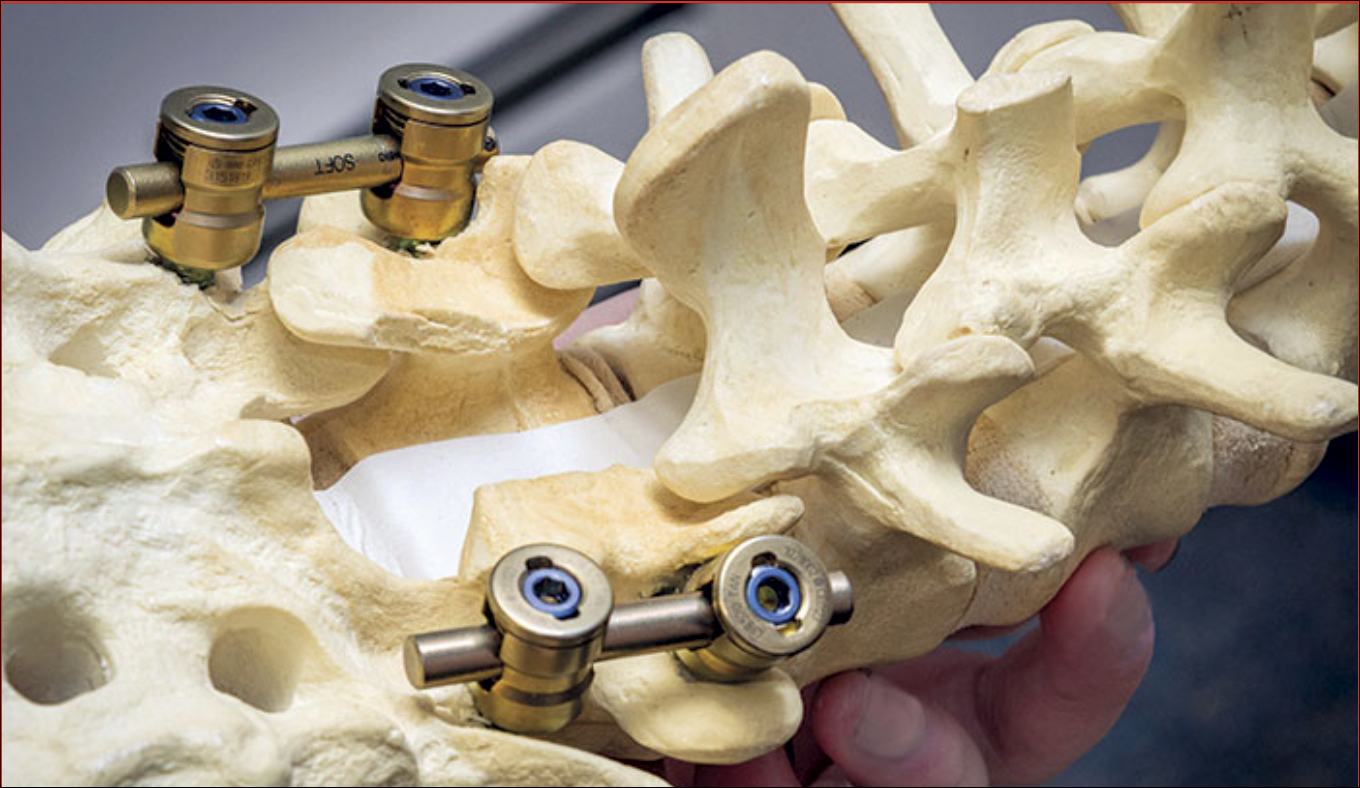
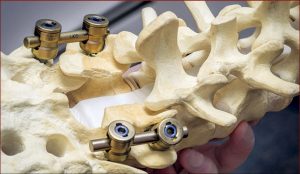
Laminectomy involves creating a safe passage in the spine of SMA patients to facilitate injection of Spinraza. (Photo courtesy of Tomasz Potaczek, MD)
Polish orthopedic surgeon Tomasz Potaczek says people with spinal muscular atrophy (SMA) who have had surgery to correct scoliosis are routinely denied access to Spinraza (nusinersen) because of the difficulty in administering the injections intrathecally (via the spinal canal).
To correct what he calls this “injustice,” Potaczek has developed a surgical technique that involves operating to correct spinal deformities, but leaving a place in the spine where there is no bone, allowing Spinraza to be injected multiple times in that same place.
“Certainty it is no way to approach the matter if we try to divide patients and force them to choose either surgery or medical treatment,” said Potaczek, who presented his findings at the 6th Polish SMA Conference in Warsaw. The Aug. 23-25 event was hosted by the nonprofit group Fundacja SMA.
Potaczek told SMA News Today in a subsequent phone interview that at least 85 percent of SMA patients will eventually develop kyphoscoliosis, or spinal deformities. Generally speaking, the more severe the SMA type, the more severe the deformity. In addition, the more oblique the pelvis is, the greater the curve progression of the spine.
Ask questions and share your knowledge of SMA in our forums.
“The main idea is that most patients with SMA will, at a certain point, require surgery for their spinal deformities. Depending on the severity of the disease itself, the onset of spinal deformity might be earlier in those very severely affected patients, and later in SMA 3 types,” he said.
“Surgery for those patients is relatively safe, and the risk of serious complications is very low. There is no age limit for surgery, though there are specific surgical techniques developed for very young or older patients.”
The problem isn’t so much with the surgery itself, but that having it could later deprive patients from getting access to Spinraza, he explained.
“Neurologists don’t want to give Spinraza to patients after spine surgery because it’s technically more demanding. They said no to these patients — many of whom were waiting for Spinraza while their spine was becoming more and more curved,” Potaczek said.
SMA exclusion rules ‘unfair,’ says doctor
Spinraza, developed by Biogen, is the only approved treatment for almost all types of SMA in many countries around the world. Doctors now inject it in into the spinal fluid — a procedure called intrathecal injection — every four months throughout a patient’s life. That allows Spinraza to easily reach the spinal cord and brain, both of which are affected in SMA.
“Normally when you do spinal surgery for deformities, you want solid fusion from the back. There’s a large amount of bone in the back of the spine where they put the needle,” Potaczek explained. “I leave a special gap in this bone to create a safe passage for the needle where there is no bone.”
No other surgeons are doing this in Poland — home to as many as 700 SMA patients — and very few, if any, take this approach in other European countries.
Potaczek said he operates on SMA patients at least once a month; so far this year, he’s performed 10 spinal surgeries on people with the disease.
“I think it’s very unfair that Spinraza is limited to patients who have no severe spine deformities or who didn’t have surgery,” he said. “Some of those patients had undergone surgery 10 years ago and have been waiting all their lives for a drug to treat SMA — and now that there is one, they’re told ‘no, it’s not for you because you had surgery.’”
U.S. researchers recently developed another method of delivering Spinraza, one involving a subcutaneous intrathecal catheter (SIC) system that connects a catheter inserted in the spinal cord to an implantable infusion port. This self-sealing silicone disk, placed under the skin, can be punctured with a special needle many times to administer a therapy.
The new approach was outlined in the study, “Preliminary Safety and Tolerability of a Novel Subcutaneous Intrathecal Catheter System for Repeated Outpatient Dosing of Nusinersen to Children and Adults With Spinal Muscular Atrophy,” which appeared in the Journal of Pediatric Orthopaedics.
Researchers implanted the SIC system in 10 SMA patients with advanced disease and spine deformities — all of them between 5 and 30 years old, and with three copies of the SMN2 gene —and evaluated its safety and tolerability over three sequential doses of Spinraza.
The device was inserted in under two hours and was well-tolerated in all patients, who stayed in the hospital, on average, for less than two and a half days.
All doses of Spinraza were administered in less than 20 minutes on the first attempt, and required no sedation, medication to relieve pain, ultrasound guidance, or respiratory precautions.
SIC delivery of Spinraza barred in EU
As promising as it may be, Potaczek said this isn’t an option in the 28-member European Union, whose European Medicines Agency (EMA) approved Spinraza in May 2017.
“As far as I know, it’s not possible in Europe because the SmPC [Summary of Product Characteristics of the EMA] prohibits the use of subcutaneous access ports in the long term,” he said. “It has to be administered through a single access route, so in Europe this is not allowed.”
Potaczek said he ends up talking about Spinraza to virtually every family of an SMA patient who comes to see him.
“For the last three years, there has never been a situation where we didn’t speak about Spinraza,” he said. “It should be available for all patients.”
Kacper Rucinski, president of Fundacja SMA and organizer of the Warsaw conference, said that because Spinraza is not a one-off treatment but has to be repeated every few weeks and then every few months, doctors preferred not to take their chances.
“Normally when you do a spinal jab, the patient has to bend. If the patient is unable to, it’s next to impossible to insert the needle between the vertebrae — especially if they were previously undergoing spinal fusion,” said Rucinski, who has a 9-year-old daughter with SMA type 2.
Nevertheless, he said, such patients are often formally excluded from drug programs.
“In some countries it is expressly stated that those who have had surgery are excluded from Spinraza,” he said. “I think they should be given a chance, and it should be up to the treating doctor, not the government, to decide whether or not to undertake the procedure.”