FAQs about SMA gene therapy
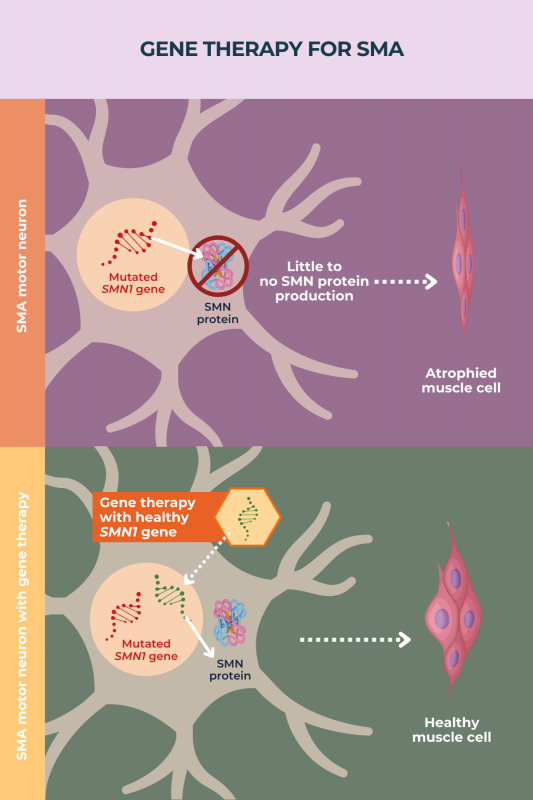
Gene therapy can slow the progression of spinal muscular atrophy (SMA), but has limited ability to reverse damage already accrued. Some babies with SMA who were given gene therapy, specifically Zolgensma, before they developed symptoms, have reported near-normal motor development, but children treated later commonly have delayed or limited motor development. To date, no gene therapy for adults with SMA has been developed.
In people with spinal muscular atrophy (SMA), chronic pain tends to arise due to mobility issues and scoliosis, or a sideways curvature of the spine, which put stress on the body. Early treatment with gene therapy can prevent disease progression, helping to improve motor development and thereby reducing the risk of developing these painful complications.
Zolgensma, the only approved gene therapy for spinal muscular atrophy (SMA), has a U.S. list price of $2.1 million. Novartis, the company that sells the therapy, offers a program called OneGene that can help commercially insured patients cover out-of-pocket costs for the therapy.
Apart from Zolgensma, there are other approved disease-modifying therapies for spinal muscular atrophy (SMA). These include Spinraza and Evrysdi. Other therapies are being investigated in clinical trials.
About one in every 50 people is a carrier for spinal muscular atrophy (SMA), meaning these individuals have one copy of the SMN1 gene that carries a disease-causing mutation. SMA will only develop if both copies of the SMN1 gene — one inherited from each biological parent — contain a disease-causing mutation. If two carriers have children, there’s a 25% chance the child will develop SMA, a 50% chance that the child would be a carrier like the parents, and a 25% chance that the child would have the disease nor be a carrier.
Related Articles

 Medically reviewed by
Medically reviewed by