Gene therapy for spinal muscular atrophy
Gene therapy is a one-time treatment strategy that’s been developed for spinal muscular atrophy (SMA), a rare genetic disorder characterized by progressive muscle weakness and atrophy. As a treatment option, it aims to correct or replace the mutated gene that causes SMA.
The disease mainly affects motor function, but often also causes speaking, swallowing, and breathing problems, along with other SMA symptoms.
What is SMA gene therapy?
Nearly all cases of SMA are caused by mutations in the gene SMN1, which provides instructions for making the SMN protein. This protein is necessary for the survival of motor neurons, the nerve cells that govern movement.
Mutations in SMN1 lead to too little SMN protein being produced, resulting in the progressive dysfunction and death of motor neurons, which ultimately gives rise to disease symptoms.
The overarching aim of gene therapy for SMA is to deliver a working copy of the SMN1 gene to neurons, particularly motor neurons, thus allowing these cells to produce enough SMN protein. As with other diseases, gene therapy in SMA is given as a one-time treatment.
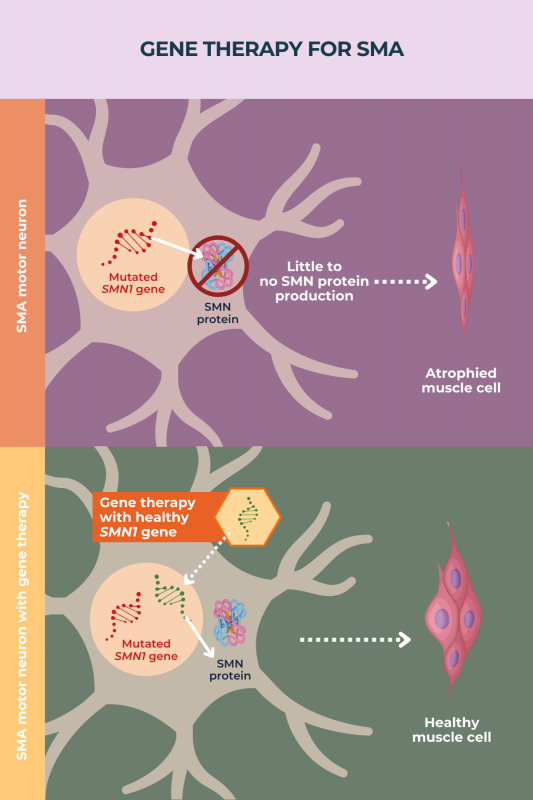
Types of gene therapy
Two gene therapies, both marketed by Novartis, are currently approved in the U.S. to treat SMA:
Both use a harmless, lab-engineered version of adeno-associated virus serotype 9 (AAV9) to deliver their genetic payload to the body’s cells.
Zolgensma
In the U.S., Zolgensma is authorized to treat patients up to age 2 with all types of SMA caused by mutations in the SMN1 gene. In the European Union, Zolgensma is approved for treating some SMA patients. The therapy also is approved in other countries, though specific indications may vary.
Itvisma
In 2025, U.S. regulators approved a second gene therapy, Itvisma, for adults and children with SMA, ages 2 and older, with a confirmed SMN1 mutation. Itvisma, formerly known as OAV101 IT, contains the same active agent as Zolgensma, but is administered via an injection into the spinal canal, or intrathecally, rather than intravenously. This direct mode of administration into the fluid surrounding the spinal cord means Itvisma can be successfully delivered to motor neurons when given at a lower dose.
How is gene therapy administered?
Zolgensma is administered via an intravenous (into-the-vein) infusion, which usually takes about one hour to complete. Itvisma is given via an intrathecal injection that lasts one to two minutes. Both are one-time treatments.
While Zolgensma uses a weight-based dosing regimen, Itvisma is given at a fixed standard dose regardless of body weight.
Starting the day before gene therapy treatment, and for at least one month afterward, patients are given a course of inflammation-suppressing medicines called corticosteroids. This is designed to reduce the risk of the immune system responding to the gene therapy like an infectious threat. The aim is to prevent inflammation that damages organs, particularly the liver. Once liver lab values have normalized and are stable, the patient can be gradually weaned off corticosteroid treatment.
Values of liver enzymes should be monitored for at least three months following treatment with Zolgensma or Itvisma. Levels of platelets — cell fragments involved in clotting — and troponin-I, a marker of heart muscle damage, also should be monitored in the months following treatment.
Benefits of gene therapy
Gene therapy is a one-time treatment specifically designed to treat the underlying genetic cause of SMA and replace the missing protein. This approach may improve the quality of life of patients and reduce the amount of treatments needed over an individual’s lifetime.
Like other disease-modifying SMA treatments, gene therapy is generally effective at slowing disease progression, but its ability to reverse damage that has already happened is limited. As such, treatment outcomes are typically more favorable when gene therapy is given as early as possible.
Zolgensma
Clinical trials have demonstrated that Zolgensma can slow the progression of SMA, significantly decreasing the risk of death or needing ventilation to help with breathing. Treatment with Zolgensma also may help normalize motor development. Some children with severe forms of SMA who were treated with Zolgensma have reached milestones such as walking independently, altering the natural course of the disease. These motor milestones were maintained for up to a decade in some cases.
However, little is known about the benefits Zolgensma may offer for adolescents or adults with SMA.
Itvisma
Itvisma’s intrathecal dosing allows it to be effective at a lower fixed dose, making it suitable for older patients.
In a clinical trial involving children with SMA type 2 who could sit but had never been able to walk independently, Itvisma led to significant improvements in motor function after a year.
A separate clinical trial also demonstrated that the therapy was able to stabilize motor function in children who had previously been treated with Spinraza (nusinersen) or Evrysdi (risdiplam).
Cost of gene therapy
Gene therapies for SMA are among the most expensive treatments in the world. In 2025, the U.S. list price of Zolgensma was $2.5 million, while Itvisma was available for $2.59 million. As one-time treatments, Zolgensma and Itvisma may offer long-term therapeutic benefits, which could potentially reduce the lifetime costs associated with SMA.
Some health insurers cover these therapies, but others do not — and companies that do provide coverage may have criteria about who qualifies for the treatment that are stricter than regulatory approvals. These criteria can include limitations on age or whether patients show symptoms.
Novartis has a patient support program that offers one-on-one assistance to families of patients prescribed Zolgensma and Itvisma. Among other services, the Zolgensma and Itvisma CopayAssist Program is available to help cover out-of-pocket costs of gene therapy for eligible patients with private insurance.
Nonprofit organizations such as the PAN Foundation also may help families affected by SMA cover costs of Zolgensma and other treatments. The foundation provides assistance of up to $4,600 per year.
SMA News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 


