A Fresh START: Life After SMA Treatment
In 2014 and 2015 respectively, Natalie and Tina received life-altering news. Their infant sons had each been diagnosed with a rare genetic disorder.
“I remember asking the doctor, ‘How much time do we have?’” said Tina. “I could tell she didn’t want to answer, but eventually, she gave it to me straight: six to 12 months. And I said, ‘My son will not be a statistic. You just wait and see.’ But as soon as I got to the car, I broke down, sobbing, screaming, crying.” Her son, Malachi, is now eight.
“Hospice was in the driveway for my baby,” said Natalie, whose son Eli will turn eight in December. “We got discharged without a diagnosis initially, and finally heard back after blood tests. It was Halloween, 2014, and I remember thinking that I just wanted him to eat a sucker, but instead, his whole world could be ripped away. It felt so out of our control.”
Malachi and Eli were diagnosed with spinal muscular atrophy, or SMA. It is a genetic disorder that leads to progressive muscle weakness, paralysis, and when left untreated in its most severe forms, permanent ventilation or death in 90% of cases by age two. SMA is caused by a nonfunctioning or nonexistent survival motor neuron 1 (SMN1) gene, which is responsible for producing SMN protein. Without enough SMN protein, motor neuron cells throughout the body lose function and die.
Natalie and Tina each worked closely with their sons’ healthcare teams to figure out what to do next. Critically, the FDA hadn’t yet approved any treatments for SMA.
“We didn’t have any other options, so I called a hospital conducting a clinical trial,” explained Natalie. “The doctor said, ‘I hear you’ve been given some pretty bad news and your son was diagnosed with SMA. I can’t make any promises, but I’d really like to try to help. When can you be here?’ And I said, ‘I’ll be there tomorrow.’”
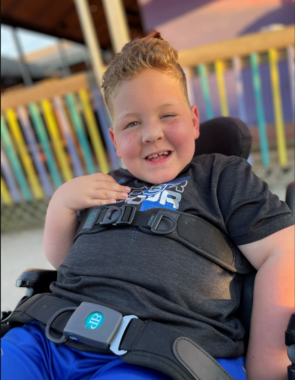
Eli

Malachi
Malachi and Eli were both enrolled in START, the first clinical trial for Zolgensma® (onasemnogene abeparvovec-xioi). Zolgensma is the only gene therapy for the treatment of SMA, and the only SMA treatment designed to address the genetic root cause of the disease by replacing the function of the missing or non-working SMN1 gene with a single, one-time dose. It was approved by the U.S. FDA on May 24, 2019.
Zolgensma can increase liver enzyme levels and cause acute serious liver injury or acute liver failure which could result in death. In clinical trials, the most common side effects were elevated liver enzymes and vomiting. Please see additional Important Safety Information below and accompanying Full Prescribing Information. Children treated with Zolgensma need to receive an oral corticosteroid starting the day before infusion, and then after infusion for about two months or longer depending on their liver function exams and labs. Children treated with Zolgensma also need baseline labs and then need to return for blood tests weekly, bi-weekly and then monthly for at least the first three months after treatment.
Eli and Malachi were among the first babies to be treated with Zolgensma. Since, more than three thousand children have received this treatment around the world.
“When Eli got dosed, I wanted to see results immediately,” said Natalie. “That is one of the hardest things I had to learn while watching my kid go through treatment. I had many disappointing days, thinking he was going backwards. But he’s just a kid, and kids just have bad days.”
Now years after their dosing days, both families are working on independence for their boys—a goal that isn’t as abstract as it may seem.
“Today, I transfer Eli to his power chair, and he pretty much does life on his own,” shared Natalie. “He doesn’t need me like he used to. At one point, I felt like I was running a race to keep him alive, but his independence has really blossomed over the last year.”
Eli plays soccer in his wheelchair and loves to go fast. His mom describes him as grateful, honest and compassionate. He skipped first grade, and he loves math.
“He just recently started helping with chores, like cleaning off the kitchen table,” explained Natalie. “He’s become so confident and capable. He really loves a challenge.”

Eli

Malachi
“We were told that Malachi would never speak, never sit unassisted. There were all these ‘nevers’ stacked against him. And now we’re working on balance and endurance with Malachi. Walking has never been the goal, but his smile when he’s standing… it brightens a whole room,” said Tina.
Malachi’s mom says he soaks knowledge in like a sponge. And he’s got passion to boot. “I won a science kit at the science fair, which is awesome. I’m really lucky at my school. I love science,” said Malachi.
“He loves to learn. He’s so in touch with his own life and surroundings, and he’s so grateful and articulate,” said Tina.
Both boys have hard days, but their families are tight knit, resilient, and ready to help—including their siblings.
“They see me adapting everything so that Malachi can be included,” said Tina. “So, they do their best to do the same in whatever they do. They even opt out of activities because they don’t know how to make it work for him.
Even when at school, Eli’s loved ones make sure he’s taken care of—two of his best friends act as his Peer Assistants, walking to classes with him and helping with anything else he might need.
“This past year of school has been the first year that I haven’t worried about him,” said Natalie. “If I had it to do all over again, I would have wiped the word worry completely out of my vocabulary.”
Year after year, these boys defy the natural history of SMA, proving to their families, their doctors and themselves that they aren’t defined by their diagnosis. They work hard in physical therapy, excel in school and impress their families every day.
“When we first got his new bike, we were riding on the trail,” explained Tina, “And he said, ‘Mom, I’m getting tired. But I’m not giving up.’ That drive makes me so happy. If I teach my kids anything, I want them to never give up,” explained Tina, “and he never does.”
Results and outcomes vary among children based on several factors, including how far their SMA symptoms progressed prior to receiving treatment.

Natalie and Eli

Tina and Malachi
Indication and Important Safety Information for ZOLGENSMA® (onasemnogene abeparvovec-xioi)
What is ZOLGENSMA?
ZOLGENSMA is a prescription gene therapy used to treat children less than 2 years old with spinal muscular atrophy (SMA). ZOLGENSMA is given as a one-time infusion into a vein. ZOLGENSMA was not evaluated in patients with advanced SMA.
What is the most important information I should know about ZOLGENSMA?
- ZOLGENSMA can increase liver enzyme levels and cause acute serious liver injury or acute liver failure which could result in death.
- Patients will receive an oral corticosteroid before and after infusion with ZOLGENSMA and will undergo regular blood tests to monitor liver function.
- Contact the patient’s doctor immediately if the patient’s skin and/or whites of the eyes appear yellowish, if the patient misses a dose of corticosteroid or vomits it up, or if the patient experiences a decrease in alertness.
What should I watch for before and after infusion with ZOLGENSMA?
- Infections before or after ZOLGENSMA infusion can lead to more serious complications. Caregivers and close contacts with the patient should follow infection prevention procedures. Contact the patient’s doctor immediately if the patient experiences any signs of a possible infection such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
- Decreased platelet counts could occur following infusion with ZOLGENSMA. Seek immediate medical attention if the patient experiences unexpected bleeding or bruising.
- Thrombotic microangiopathy (TMA) has been reported to generally occur within the first two weeks after ZOLGENSMA infusion. Seek immediate medical attention if the patient experiences any signs or symptoms of TMA, such as unexpected bruising or bleeding, seizures, or decreased urine output.
- There is a theoretical risk of tumor development with gene therapies such as ZOLGENSMA. Contact the patient’s doctor and Novartis Gene Therapies, Inc. (1-833-828-3947) if a tumor develops.
What do I need to know about vaccinations and ZOLGENSMA?
- Talk with the patient’s doctor to decide if adjustments to the vaccination schedule are needed to accommodate treatment with a corticosteroid.
- Protection against influenza and respiratory syncytial virus (RSV) is recommended and vaccination status should be up-to-date prior to ZOLGENSMA administration. Please consult the patient’s doctor.
Do I need to take precautions with the patient’s bodily waste?
Temporarily, small amounts of ZOLGENSMA may be found in the patient’s stool. Use good hand hygiene when coming into direct contact with patient body waste for one month after infusion with ZOLGENSMA. Disposable diapers should be sealed in disposable trash bags and thrown out with regular trash.
What are the possible or likely side effects of ZOLGENSMA?
The most common side effects that occurred in patients treated with ZOLGENSMA were elevated liver enzymes and vomiting.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report suspected side effects by contacting the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch, or Novartis Gene Therapies, Inc. at 1-833-828-3947.
Please see the Full Prescribing Information.
© 2023 Novartis Gene Therapies, Inc.
Bannockburn, IL 60015
US-ZOL-23-0088 10/2023
###