The Road Ahead
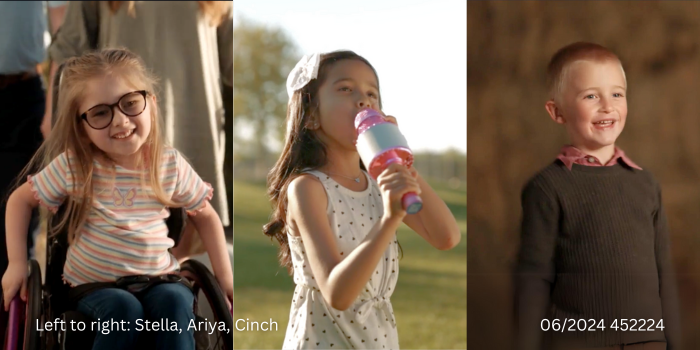
In the fall, Stella, Ariya and Cinch are all starting kindergarten. Spoiler alert: they’re excited.
Their parents are excited, too, and nervous – the transition to school is a big milestone for any child, but going to kindergarten is extra special for these little ones. Years ago, these parents weren’t so sure their children would survive to see their second birthdays.
Stella, Ariya and Cinch all have spinal muscular atrophy, or SMA. There are different types of SMA, and every case is different. Without treatment, this devastating genetic disorder can lead to progressive, irreversible muscle weakness and paralysis and, in its most severe forms, permanent ventilation or death in 90% of cases by age two. It’s caused by a missing or nonfunctioning SMN1 gene, which produces the protein needed by neurons to control muscles. All three of their diagnosis and treatment journeys are different, but it all started five years ago.
“When we were first introduced to SMA, we were introduced to this horrible, devastating disease,” said Stella’s mom, Samantha. Her daughter had appeared developmentally typical on initial reflex exams, but within three weeks, doctors could tell that something was causing Stella to weaken, losing most of the movement in her legs. After additional appointments, at one month old, Stella was diagnosed with SMA Type 1. Her neurologist said he’d never seen a baby regress so quickly.
At around five months old, Ariya’s parents, Mitul and Chelsey, noticed similar symptoms. Ariya wasn’t putting weight on her legs, so her parents knew something wasn’t quite right. Several doctor visits later, she was diagnosed with SMA Type 2.
Cinch was diagnosed with SMA after being screened as a newborn. His parents, Alex and Amber, had never heard of the disease. “I called Alex,” explained Amber. “He worked just up the road, so he rushed home. It was a shock. Cinch has three copies of the SMN2 gene, which is the backup for the SMN1 gene.”
After discussing the two treatment options that were available at the time with their doctor, Samantha and her husband decided to pursue gene therapy. “It was a no-brainer for us to choose ZOLGENSMA,” she explained.
On May 24, 2019, the U.S. Food & Drug Administration (FDA) approved the one-time gene therapy ZOLGENSMA® (onasemnogene abeparvovec-xioi). ZOLGENSMA is the first and only treatment designed to replace the function of the missing or non-functioning SMN1 gene by delivering a new, working copy of the SMN gene, allowing a child’s body to produce its own SMN protein.
ZOLGENSMA can increase liver enzyme levels and cause acute serious liver injury or acute liver failure which could result in death. In clinical trials, the most common side effects were elevated liver enzymes and vomiting. Please see additional Important Safety Information below and accompanying Full Prescribing Information. Children treated with ZOLGENSMA need to receive an oral corticosteroid starting the day before infusion, and then after infusion for about two months or longer depending on their liver function exams and labs. Children treated with ZOLGENSMA also need baseline labs and then need to return for blood tests weekly, bi-weekly and then monthly for at least the first three months after treatment.
The efficacy of ZOLGENSMA has been observed in symptomatic and presymptomatic patient populations across clinical trials. Additionally, the safety of ZOLGENSMA is being observed in post-treatment, 15-year long-term follow-up studies. Clinical research is ongoing to broaden knowledge on safe and effective treatment with ZOLGENSMA across ages, weight ranges, SMN2 copy number and more.
Within six months of FDA approval, Stella, Ariya and Cinch had each received their one-time dose.
“Cinch was just over a month old when he was treated with ZOLGENSMA, and he slept through the whole process of getting it,” said Alex.
“Still, he has not shown a single sign of SMA,” explained Amber.
The couple dreams that one day, Cinch will saddle broncs in the rodeo like Alex did as a child. For now, he sticks to riding the family’s tractors and feeding the baby cows on their ranch. “Because of ZOLGENSMA, we get to enjoy and hang out with him every day and do what we love, and he’s learning to love it too,” said Amber.
But the path forward for kids and families with SMA hasn’t always been so hopeful.
Mitul recalls the period after Ariya’s diagnosis clearly: “I’ll never forget where I was at that time. I can remember the weather on that day and what I was wearing and what I was doing. That was the bleakest moment in our lives, when we thought that we were going to lose our child.”
“There was little reassurance that we could offer families even ten years ago,” said Dr. Sandra P. Reyna, Executive Medical Director of SMA, Global Medical Affairs, at Novartis. She has worked with patients with SMA and their families for close to two decades.
“It was beyond difficult to watch families go through that experience, knowing that the best they could hope for was for the progression to slow, and to manage the symptoms,” she said. “Now, with several FDA-approved treatments, families have options to best meet the needs of their child and circumstances.”
Since receiving ZOLGENSMA, Ariya and Stella have both started an additional SMA treatment.
Though results differ patient to patient, especially based on disease progression, treatment enables many families to experience moments with their child that they never thought possible, and to feel excited for their child’s future.
Stella gets around in her wheelchair or by scooting on the floor. She’s working on bearing weight on her legs, and her parents think she’ll play adaptive sports, probably soccer. She is anything but limited by her “wheels.”
“Stella knows she has a disability, but she knows that she can figure out how to do things her own way,” said Samantha. “Whatever achievements she wants to reach, we’ll be there cheering for her.”
“Our view on disability has changed completely within the last five years,” explained Samantha. “She is a really good advocate for herself. And that’s something that we’ve always been very proud of and always encouraged.”
“It’s kind of like the clouds are lifted, the sun is shining. Ariya is a happy kid. She’s able to do all these things,” said Mitul. Ariya is brave and a problem-solver, always surprising her parents with new skills. “She knows how to navigate her world. Kids are pretty awesome, adapting to their realities.”
Every child with SMA is different, and every family’s journey through diagnosis and treatment is unique. But Stella, Cinch and Ariya all have one important thing in common – with treatment, their futures are bigger and brighter than ever.
Results and outcomes vary among children based on several factors, including how far their SMA symptoms have progressed prior to receiving treatment.
+++
Indication and Important Safety Information for ZOLGENSMA® (onasemnogene abeparvovec-xioi)
What is ZOLGENSMA?
ZOLGENSMA is a prescription gene therapy used to treat children less than 2 years old with spinal muscular atrophy (SMA). ZOLGENSMA is given as a one-time infusion into a vein. ZOLGENSMA was not evaluated in patients with advanced SMA.
What is the most important information I should know about ZOLGENSMA?
- ZOLGENSMA can increase liver enzyme levels and cause acute serious liver injury or acute liver failure which could result in death.
- Patients will receive an oral corticosteroid before and after infusion with ZOLGENSMA and will undergo regular blood tests to monitor liver function.
- Contact the patient’s doctor immediately if the patient’s skin and/or whites of the eyes appear yellowish, if the patient misses a dose of corticosteroid or vomits it up, or if the patient experiences a decrease in alertness.
What should I watch for before and after infusion with ZOLGENSMA?
- Infections before or after ZOLGENSMA infusion can lead to more serious complications. Caregivers and close contacts with the patient should follow infection prevention procedures. Contact the patient’s doctor immediately if the patient experiences any signs of a possible infection such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
- Decreased platelet counts could occur following infusion with ZOLGENSMA. Seek immediate medical attention if the patient experiences unexpected bleeding or bruising.
- Thrombotic microangiopathy (TMA) has been reported to generally occur within the first two weeks after ZOLGENSMA infusion. Seek immediate medical attention if the patient experiences any signs or symptoms of TMA, such as unexpected bruising or bleeding, seizures, or decreased urine output.
- There is a theoretical risk of tumor development with gene therapies such as ZOLGENSMA. Contact the patient’s doctor and Novartis Gene Therapies, Inc. (1-833-828-3947) if a tumor develops.
What do I need to know about vaccinations and ZOLGENSMA?
- Talk with the patient’s doctor to decide if adjustments to the vaccination schedule are needed to accommodate treatment with a corticosteroid.
- Protection against influenza and respiratory syncytial virus (RSV) is recommended and vaccination status should be up-to-date prior to ZOLGENSMA administration. Please consult the patient’s doctor.
Do I need to take precautions with the patient’s bodily waste?
Temporarily, small amounts of ZOLGENSMA may be found in the patient’s stool. Use good hand hygiene when coming into direct contact with patient body waste for one month after infusion with ZOLGENSMA. Disposable diapers should be sealed in disposable trash bags and thrown out with regular trash.
What are the possible or likely side effects of ZOLGENSMA?
The most common side effects that occurred in patients treated with ZOLGENSMA were elevated liver enzymes and vomiting.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report suspected side effects by contacting the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch, or Novartis Gene Therapies, Inc. at 1-833-828-3947.
Please see the Full Prescribing Information.
© 2024 Novartis Gene Therapies, Inc.
Bannockburn, IL 60015
06/2024 452224