FAQs about SMA treatment
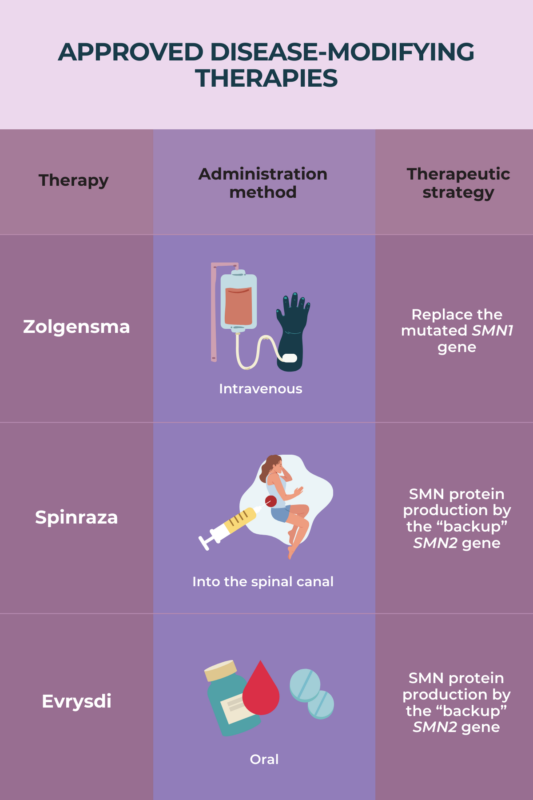
Gene therapy for spinal muscular atrophy (SMA) — Zolgensma and Itvisma are the available options — involves using a modified virus to deliver a healthy copy of SMN1, the faulty gene in SMA, to patients. This is expected to significantly slow the progressive muscle weakness that characterizes the disease.
Among several therapeutic approaches now available, disease-modifying therapies (DMTs) show the greatest effects in terms of symptom reduction in spinal muscular atrophy (SMA). DMTs are able to slow or even halt disease progression. The therapies can help children with SMA achieve otherwise unattainable motor milestones, and help older patients improve or maintain motor, breathing, and swallowing skills.
The cost of spinal muscular atrophy or SMA treatment is high, and depending on the insurance plan, some companies may provide coverage for disease-modifying therapies, while others won’t. Patients or caregivers should talk to their healthcare team and insurance providers about access to SMA treatments. Due to the Affordable Care Act in the U.S., denials or restrictions in health insurance, or higher premium rates because of a preexisting condition such as SMA, are prohibited.
To date, there is limited evidence from direct comparisons between disease-modifying therapies for spinal muscular atrophy (SMA). As such, no strong conclusions can be drawn about which treatment may be more effective. Evidence suggests that starting treatment early, especially prior to symptom onset, is more critical than DMT choice in achieving the best outcomes. SMA patients and/or their parents should talk to their healthcare team about eligibility for SMA treatments, and the potential risks and benefits of each therapy.
 Medically reviewed by
Medically reviewed by