Spinraza (nusinersen) for spinal muscular atrophy
What is Spinraza for SMA?
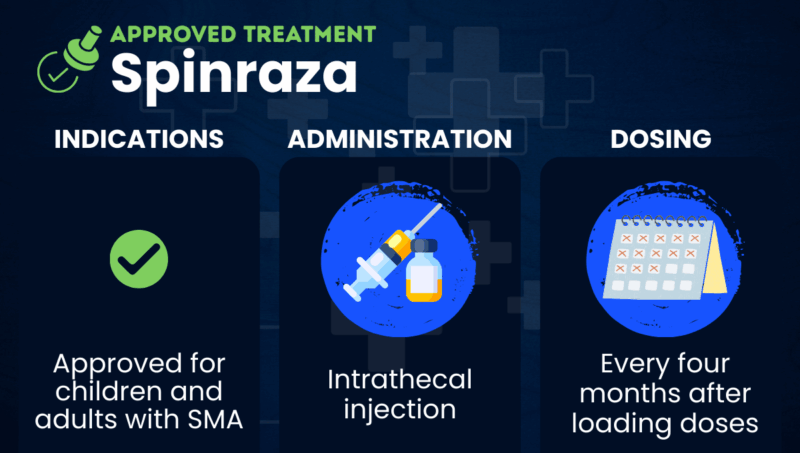
Spinraza (nusinersen) is an injection therapy widely approved for treating adults and children with spinal muscular atrophy (SMA). It was the first therapy approved to target the underlying causes of SMA.
Mutations in the SMN1 gene, which contains instructions for cells to produce the survival motor neuron (SMN) protein, cause SMA. SMN is essential for the health of the specialized nerve cells that control muscle function, called motor neurons. Without functional SMN, these neurons sicken and die, leading to symptoms of progressive muscle wasting and weakness.
Humans also have a backup SMN-producing gene, SMN2, which produces less functional protein than SMN1 due to a natural process called alternative splicing.
Spinraza contains a short strand of genetic material, called an antisense oligonucleotide, which is designed to correct SMN2 splicing, allowing the body to produce more functional SMN.
The therapy is administered via an injection into the spinal canal, called an intrathecal injection. Biogen markets it.
Therapy snapshot
| Brand name | Spinraza |
| Chemical name | Nusinersen |
| Usage | Used to maintain or improve motor function in SMA |
| Administration | Intrathecal injection |
Who can take Spinraza?
Spinraza is approved in the U.S. for the treatment of adults and children with SMA.
It is also approved in over 70 countries, including in Canada and countries of the European Union (EU), although specific indications may vary.
Spinraza’s U.S. prescribing information doesn’t list any contraindications or reasons to avoid using Spinraza.
How is Spinraza administered?
Healthcare professionals administer Spinraza via intrathecal injection. The injection is usually performed under anesthesia and takes 1 to 3 minutes. People with spinal abnormalities and very young patients may require ultrasound or other imaging to guide the injection.
In the U.S., the recommended dose of Spinraza is 12 mg, regardless of weight or age. There is an initial loading period of four doses: the first three every 14 days, and the fourth 30 days later. After that, maintenance doses are given every 4 months or 3 times a year.
In the EU and Japan, a higher dose regimen is also approved. It involves two 50 mg loading doses given two weeks apart, followed by 28 mg maintenance doses every four months. This regimen is currently under review with U.S. regulators.
Spinraza in clinical trials
Three clinical trials mainly supported the U.S. approval of Spinraza.
- The Phase 3 ENDEAR trial (NCT02193074) involved 121 infants with symptomatic SMA who received either Spinraza at an equivalent to its approved dose or sham injections. While 51% of the Spinraza group showed improved motor development, none of the infants in the sham group did. Those on Spinraza were also 47% less likely to die or require permanent breathing support.
- The Phase 3 CHERISH trial (NCT02292537) enrolled 126 symptomatic children with later-onset SMA who were unable to walk independently. Over half (57%) of the group receiving the approved dose of Spinraza had clinically meaningful improvements in motor function, compared with 26% in the sham group. Younger children and children who received treatment soon after symptom onset showed the greatest improvements.
- The Phase 2 NURTURE trial (NCT02386553) tested Spinraza in 25 infants with disease-causing mutations but no symptoms. After eight years of treatment with the approved regimen of Spinraza, all participants could sit independently, and most could stand and walk without support — milestones they likely wouldn’t have reached without treatment.
A Phase 2/3 trial, DEVOTE (NCT04089566), supported the EU approval of the high-dose Spinraza regimen. It compared standard and high-dose Spinraza across the main SMA types. The treatment was found to have motor benefits for participants who had never before received Spinraza, as well as those who transitioned from the standard Spinraza regimen.
The ongoing Phase 3 ASCEND trial (NCT05067790) is testing high-dose Spinraza in participants ages 15 to 50 who previously received treatment with the SMA therapy Evrysdi (risdiplam).
The Phase 4 RESPOND trial (NCT04488133) tested Spinraza in children who didn’t respond optimally to the gene therapy Zolgensma (onasemnogene abeparvovec-xioi). After nearly 10 months of treatment, participants’ average motor function improved.
Spinraza side effects
Among patients with infantile-onset SMA, the most common side effects of Spinraza are:
- respiratory infection
- constipation
For patients with later-onset SMA, the most common side effects are:
- fever
- headache
- vomiting
- back pain
Spinraza also comes with warnings for potentially serious side effects, including:
- blood clotting abnormalities, which could cause bleeding complications
- kidney toxicity
Before starting Spinraza and before each dose, patients will undergo laboratory tests to monitor for these events.
SMA News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 


